Combination Reactions:
What is a combination reaction:
A combination reaction is a general category of chemical reactions. It is the reaction between a metal and a non-metal reactant to produce an ionic solid.
EXAMPLE:
An example of a combination would be Magnesium Oxide. Magnesium oxide is a combination of Magnesium metal and Oxygen to create a white ash like substance. Here is an example of The reaction.
Magnesium Oxide Reaction and explanation.
Decomposition Reaction:
Displacement Reactions:
In a displacement reaction...
A more reactive metal displaces a less reactive metal.
Exchange Reactions:
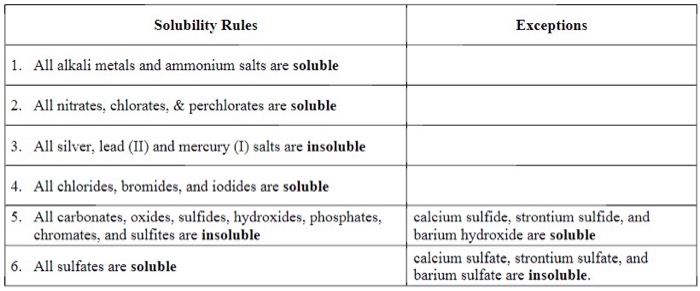
An exchange reaction occurs between compounds that, when written as a molecular equation, appear to involve the exchange of parts between the two reactants. An exchange reaction will occur when ions in solution form insoluble products
Soluble= Disolves
Insoluble= Does Not Disolve
No comments:
Post a Comment
Note: only a member of this blog may post a comment.